The Department of Justice is generally relatively transparent about its fraud prevention and False Claims Act enforcement priorities. They generally focus on broad issues that affect the lives of millions upon millions of Americans, such as the Medicare Advantage program (which covers over half of Medicare beneficiaries), unsafe medical devices, and kickbacks in healthcare. But we’ve noticed a much more specific trend emerging over the past several years: the fraudulent misbilling of electroacupuncture devices (known as peri-auricular stimulation devices or P-stims) taped to patients’ ears as surgical implants inserted into patients’ backs, with two enforcement actions coming just in the past few weeks.
What is a P-Stim?
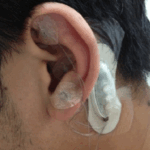 Marketed under brand names such as ANSiStim, E-pulse, Stivax, and NeuroSti, P-stim devices are used for electroacupuncture, usually to combat and prevent chronic pain. A P-stim consists of a small electronic stimulator with small acupuncture needles on the end. The stimulator is placed behind a patient’s ear, and the needle pierces the skin on the front side of the ear. The device is taped into place and generally remains with a patient for a period of five days. After five days, the device is thrown away, and the patient may get a new one.
Marketed under brand names such as ANSiStim, E-pulse, Stivax, and NeuroSti, P-stim devices are used for electroacupuncture, usually to combat and prevent chronic pain. A P-stim consists of a small electronic stimulator with small acupuncture needles on the end. The stimulator is placed behind a patient’s ear, and the needle pierces the skin on the front side of the ear. The device is taped into place and generally remains with a patient for a period of five days. After five days, the device is thrown away, and the patient may get a new one.
The picture to the left shows what a P-stim typically looks like.
The Billing Scheme
Healthcare providers generally bill the Medicaid and Medicare programs by submitting a reimbursement code, known as a common procedure terminology, or CPT, code. The CPT code at the center of this fraud scheme is L8679, for which the government pays between $5,000 and $7,500 per occurrence.
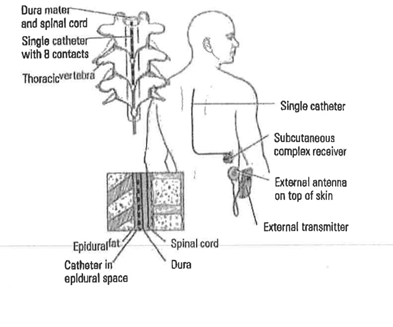 L8679 is defined as “implantable neurostimulator, pulse generator, any type” and is the billing code properly used for implanting medical devices that stimulate a patient’s spine. Like the P-Stim, these devices are also used to treat chronic pain. Unlike the P-Stim, the devices under L8679 are not taped to a patient’s ear, instead, they are surgically implanted into (or near) a patient’s spine under anesthesia, a much more complicated, and expensive, procedure.
L8679 is defined as “implantable neurostimulator, pulse generator, any type” and is the billing code properly used for implanting medical devices that stimulate a patient’s spine. Like the P-Stim, these devices are also used to treat chronic pain. Unlike the P-Stim, the devices under L8679 are not taped to a patient’s ear, instead, they are surgically implanted into (or near) a patient’s spine under anesthesia, a much more complicated, and expensive, procedure.
To the left is a diagram of a device that would be properly billed under L8679. It is clear to any layperson that this is a very different medical device from the P-Stim. This image is taken from a complaint the State of Tennessee filed against a psychiatrist under that state’s Medicaid False Claims Act.
Also, unlike the P-Stim, devices billed under L8679 are cover by Medicare, Medicaid, and TRICARE. None of these programs cover acupuncture devices like P-Stim, nor do they reimburse for P-Stim as a neurostimulator or an implantation of neurostimulator electrodes. Meaning that any Medicare, Medicaid, or TRICARE reimbursement for these devices is improper.
Data Analysis and AI
One notable thing about the deluge of enforcement actions in this space is that relatively few of them are initiated by whistleblowers. That’s somewhat unique under the FCA, where over 70% of recoveries result from whistleblower-initiated cases. This is explained by the government’s use of data analyses to uncover patterns that are likely to be fraudulent. As AI gets better and easier to use, we expect this pattern to continue.
Recent Enforcement Actions
In the past few weeks alone, there have been two enforcement actions concerning the improper billing of acupuncture devices. This follows a busy several years of the government pursuing similar theories. The defendants have varied widely, both geographically and by the type of medicine they practice:
Key Takeaways
Combatting fraudulent P-Stim billing is clearly an area of enforcement focus for the Department of Justice.
The current judgments settlements are likely just the tip of the iceberg, and P-Stim fraud is likely endemic in our system. Whistleblowers should take the government’s enforcement trend as a call to action: if you see P-Stim fraud, say something; you can help save taxpayers millions. The attorneys at Whistleblower Partners have extensive experience representing whistleblowers in cases involving healthcare fraud. If you would like more information or wish to speak to an attorney at Whistleblower Partners, please contact us for a confidential consultation.